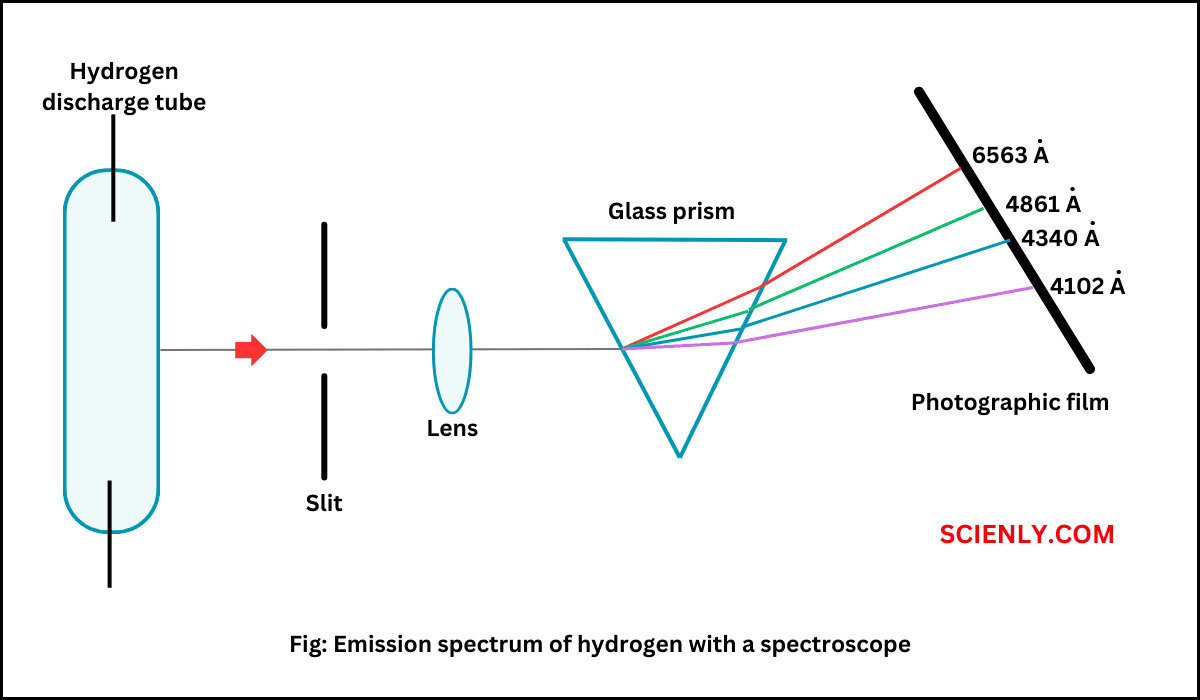
Bohr’s Atomic Model: Postulates
In this chapter, we will explore Bohr’s atomic model and its postulate. We will also see the derivation of radius of Bohr’s orbit, velocity of an electron, and energy of an electron in each orbit. Rutherford’s nuclear model of the…