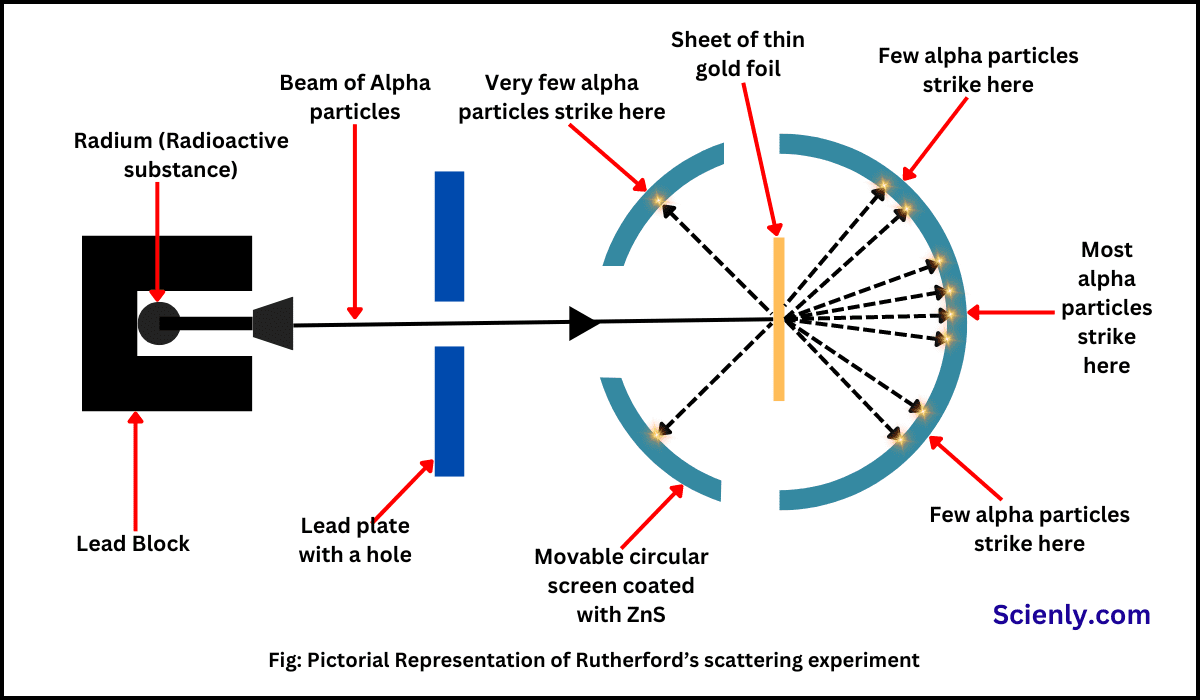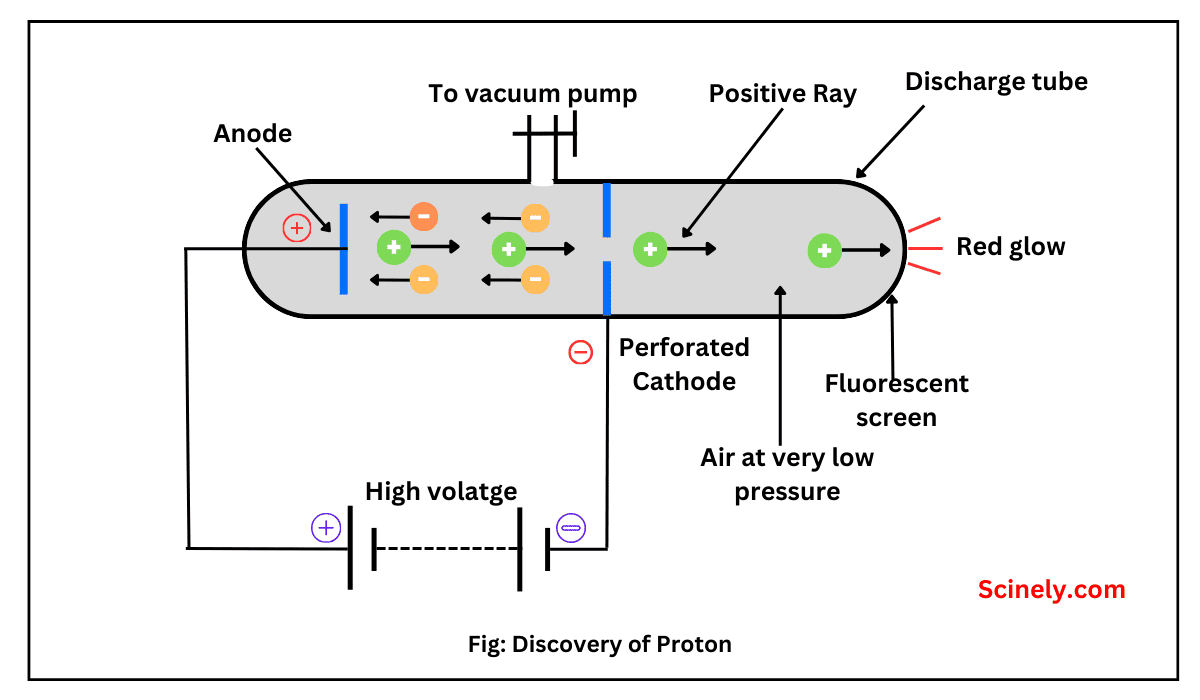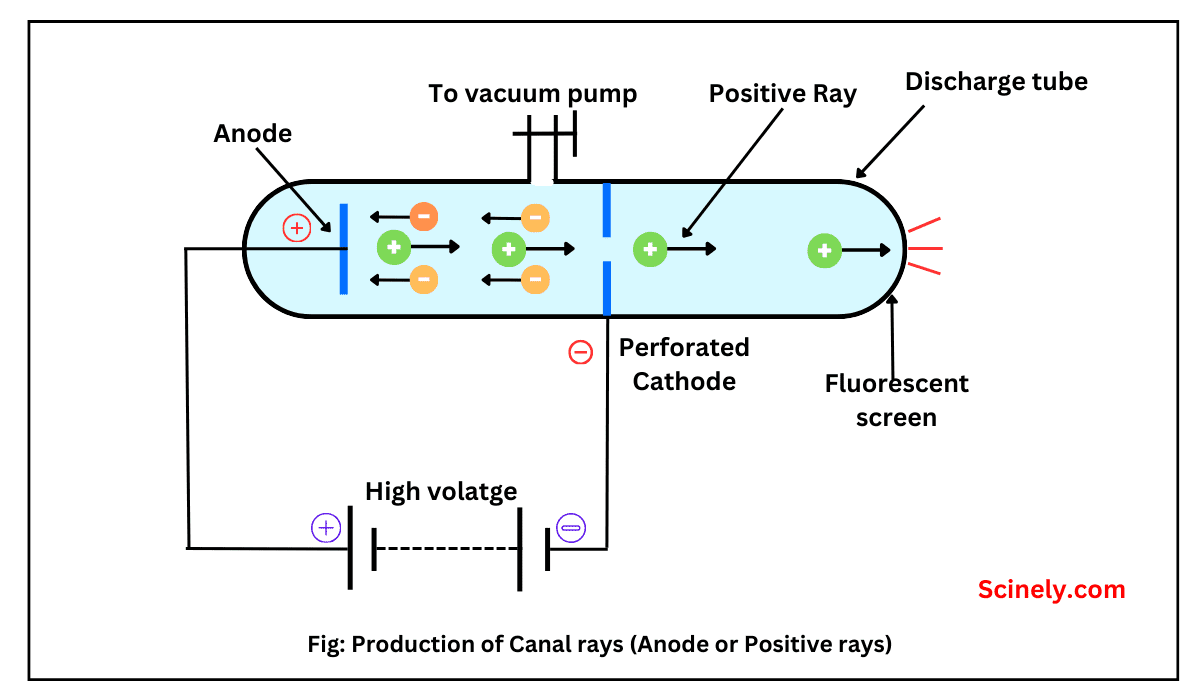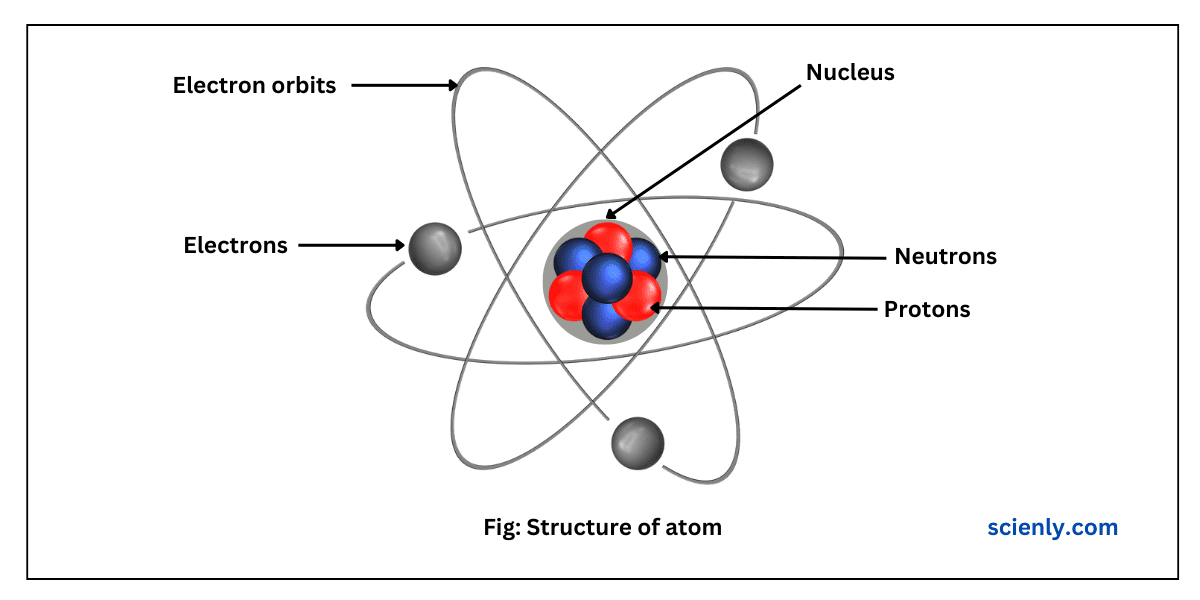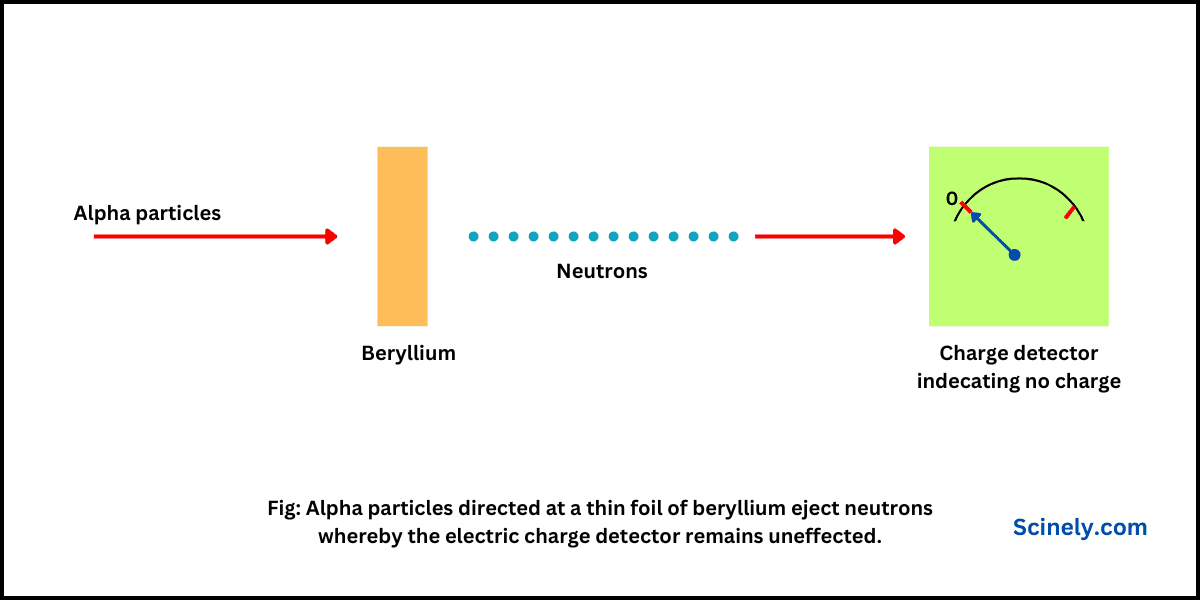
Discovery of Neutron: Chadwick’s Experiment
In this chapter, we will understand the discovery of neutron discovered by James Chadwick in 1932. Up to 1932, it was considered that an atom is composed of only electrons and protons. Since electrons have negligible mass, scientists considered that…