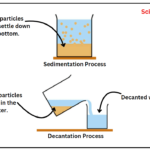
Online STEM Courses, Tutorials and Competitive Exam Preparation
Welcome to Scienly – your trusted platform to start learning.
Build a strong foundation in Science, Technology, Engineering, and Mathematics (STEM) with easy-to-understand tutorials and solved examples. Prepare for IIT JEE, NEET, SSC, UPSC, Banking, and other competitive exams with expert courses, study materials, quizzes, and notes. And the best part? Everything is completely free!
Popular Tutorials for Competitive Exams Preparation
At Scienly, we have included the most popular tutorials to help you learn various STEM and general knowledge subjects. Whether you’re just starting your learning, a college student, or an aspirant preparing for competitive exams like SSC, RRB, or UPSC, we recommend exploring these tutorials and quizzes to succeed.
General Knowledge
Science Quiz
Biology
Coding