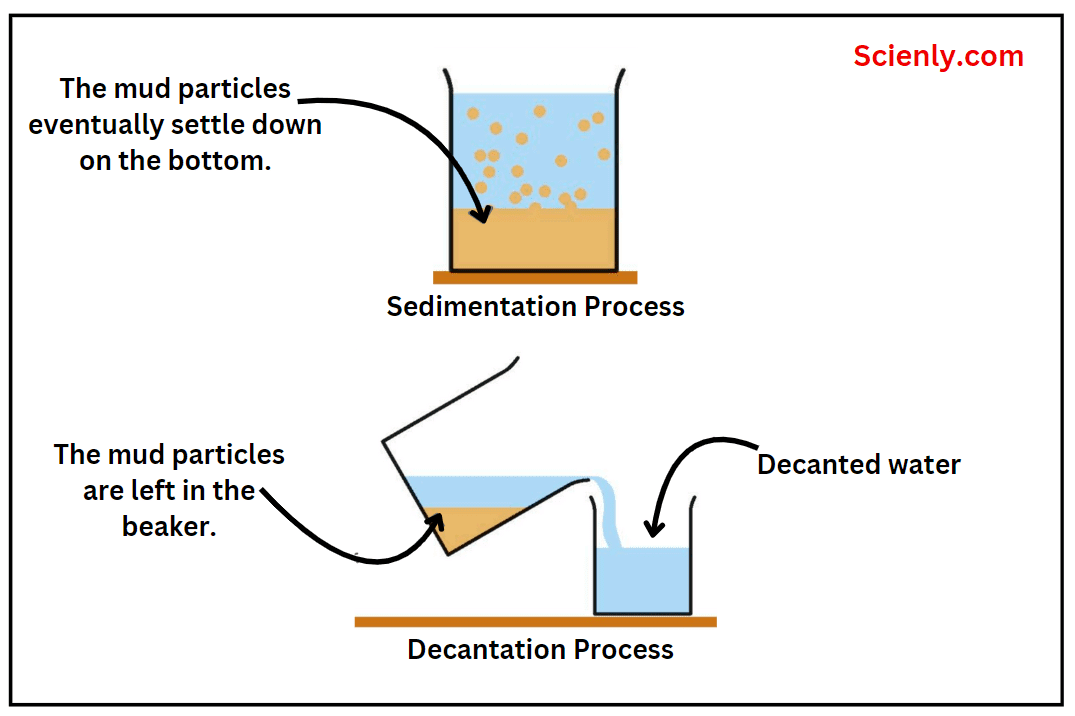
Laws of Chemical Combination with Examples
The laws which deal with the composition of substances or compounds by mass or by volume are called laws of chemical combination or laws of stoichiometry. These laws are experimental laws which have been prepared by scientists based on performing…